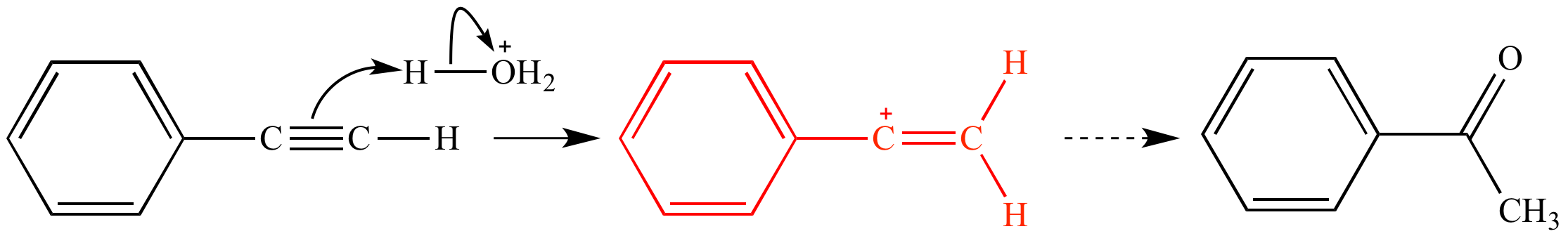
The term carbocation can be defined as an ion containing a positively charged carbon atom.
Vinyl carbocation definition.
Stability of carbocation intermediates.
The vinyl cation is a carbocation with the positive charge on an alkene carbon.
More generally a vinylic cation is any disubstituted trivalent carbon where the carbon bearing the positive charge is part of a double bond and is sp hybridized.
Because of the high s character of the orbital the positive charge resides closer to the positively charged nucleus which makes it a particularly high energy cation.
Allylic carbon atom can form stable carbocations due to electron delocalization.
General vinylic carbocation structure.
Vinylic carbocations are very unstable due to lack of p character.
The general formula for vinyl group is r ch ch 2 in which both carbon atoms are bonded with double bond and r is attached at vinylic position.
A carbocation may have one or more positive charges.
The carbocation carbon has sp hybridization.
Its empirical formula is c 2 h 3.
A vinylic carbocation which has an empirical formula of c h is a carbocation that has a positive charge only on the alkene carbon atom.
Carbocation refers to the whole molecule not only the positively charged carbon atom.
Therefore carbocations are very often reactive.
Allylic carbon meaning the double bonded carbon atoms can be classified as vinylic and allylic carbon atoms.
Since both carbon atoms form a double covalent bond so both are sp 2 hybridized.
Vinylic compounds can produce vinylic polymers such as pvc pvf pvac etc.
We know that the rate limiting step of an s n 1 reaction is the first step formation of the this carbocation intermediate.
In the absence of geometric constraints most substituted vinyl cations carry the formal positive charge on an sp hydridized carbon atom of linear geometry.
A carbocation in which the carbon atom having the open octet and positive formal charge is part of a carbon carbon double bond.
Any trivalent disubstituted carbon is generally a vinylic carbocation in which the carbon atom which is bearing the positive charge is found to be double bonded and will always exist as sp hybridized.
The vinyl cation puts positive charge at an sp hybridized carbon atom.