Acid catalyzed hydration of phenyl acetylene a terminal alkyne involves a vinylic carbocation intermediate.
Vinyl carbocation resonance.
Any trivalent disubstituted carbon is generally a vinylic carbocation in which the carbon atom which is bearing the positive charge is found to be double bonded and will always exist as sp hybridized.
Stability of carbocation intermediates.
This is very very unstable and ranks under a methyl carbocation in stability.
A vinyl cation is a positively charged molecule a cation where the positive charge is located on a vinyl group ch ch2.
This organic chemistry video tutorial explains how to determine which carbocation is most stable.
We know that the rate limiting step of an s n 1 reaction is the first step formation of the this carbocation intermediate.
Carbon with two other atoms attached prefers sp hybridization and a linear geometry.
It provides plenty of examples including allylic and vinyli.
The rate of this step and therefore the rate of the overall substitution reaction depends on the activation energy for the process in which the bond between the carbon and the leaving group breaks and a carbocation forms.
We know that the rate limiting step of an s n 1 reaction is the first step formation of the this carbocation intermediate.
Stability of carbocation intermediates.
The hybridization of a vinyl carbocation is sp hybirdized.
The atoms must remain fixed in all resonance forms.
A vinylic carbocation which has an empirical formula of c h is a carbocation that has a positive charge only on the alkene carbon atom.
Resonance forms differ only by the placement of electrons no one resonance form.
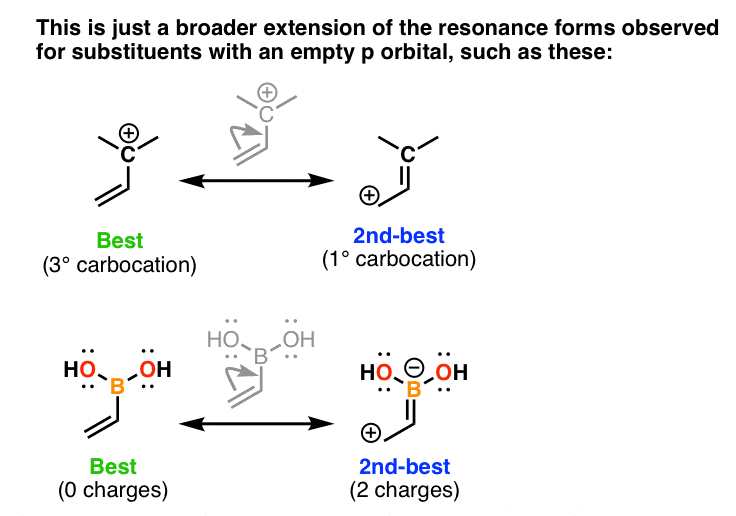
Allylic carbocations carbocation with a vinyl group as a substituent next to a double bond cc c 221 allyl carbocations are stabilized by resonance cc c cc c c c c cc c cc c recall from chapter 1 8.
The vinyl cation is a carbocation with the positive charge on an alkene carbon.
A vinyl carbocation has a positive charge on the same carbon as the double bond.
The rate of this step and therefore the rate of the overall substitution reaction depends on the activation energy for the process in which the bond between the carbon and the leaving group breaks and a carbocation forms.
In the first mechanism step the alkyne is protonated by hydronium ion a strong acid to produce a resonance stabilized secondary vinylic carbocation shown in red.
The vinyl cations are less stable due to the difference in hybridization of the carbon bearing.
Allylic carbocations are able to share their burden of charge with a nearby group through resonance.