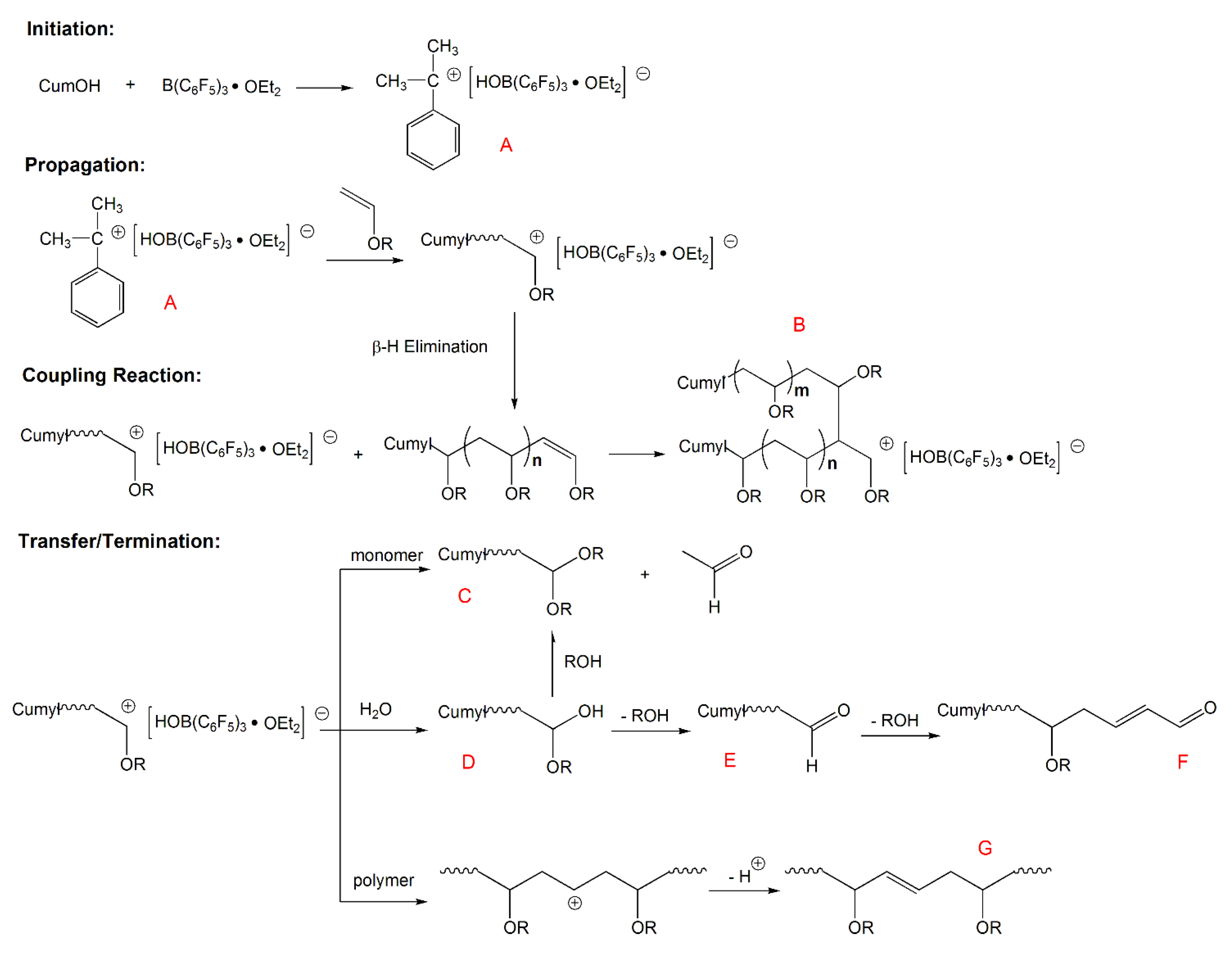
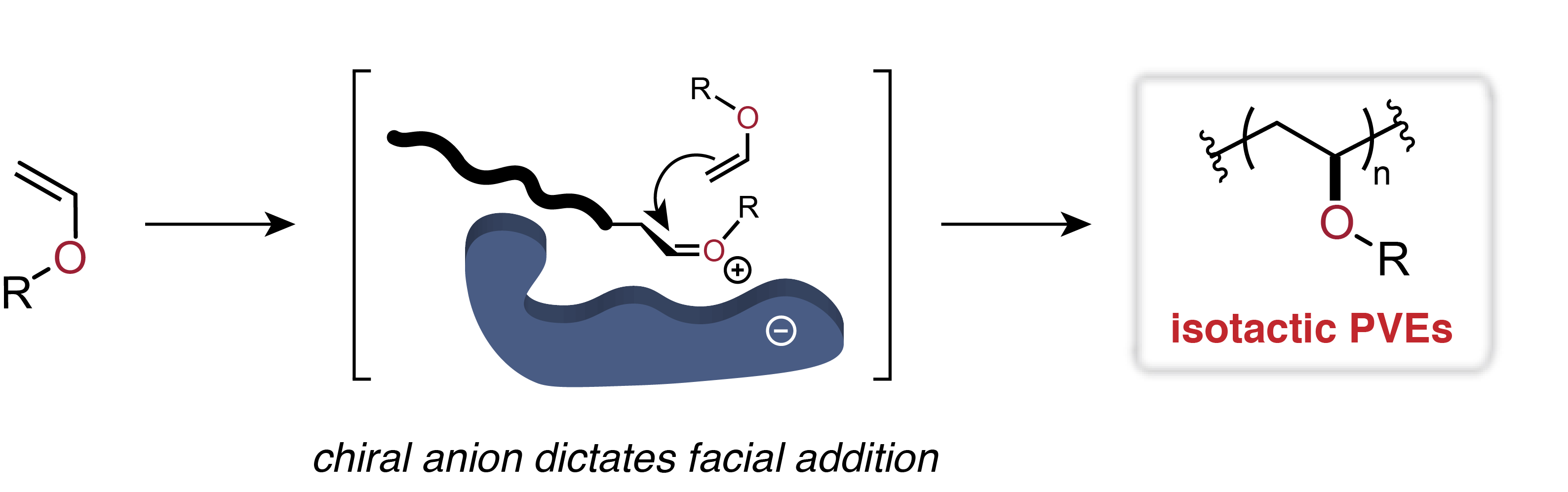
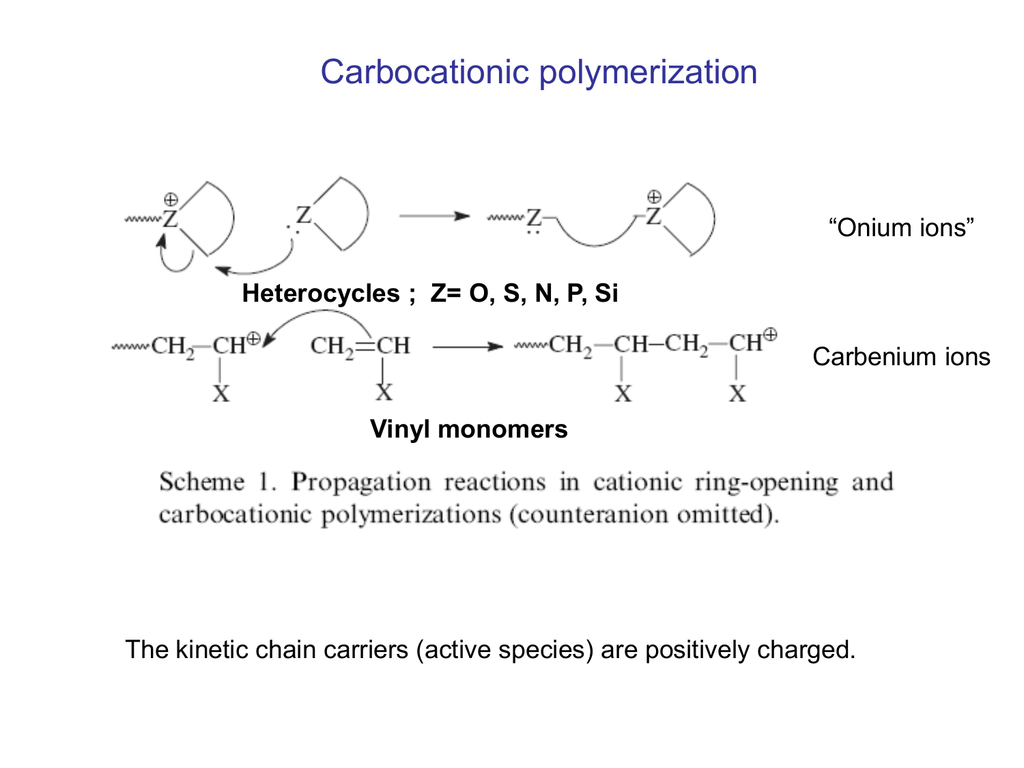
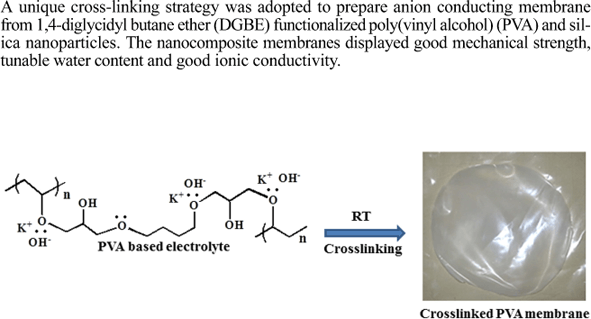
Previous approaches toward stereoselective vinyl ether polymerizations have exclusively relied on chain end control to dictate facial addition to the propagating oxocarbenium ion which has provided access to isotactic pves in specific cases but is not general to alkyl vinyl ether monomers as a class 13 19.
Vinyl ether anion.
Vinyl ether oligomers with reactive end groups were prepared by alkylation of silyl enol ethers during propagation.
Skotheim original assignee moltech corporation priority date the priority date is an assumption and is not a legal conclusion.
Di ethylene glycol divinyl ether 99.
In the current approach visible light irradiation of dimanganese decacarbonyl mn 2 co 10 in the presence of an alkyl bromide results in the formation of carbon centered radicals the photochemically generated radicals were then oxidized by diphenyliodonium ions to the corresponding cations.
Etoch ch 2 buli etoc li ch 2 buh toxicity.
Di ethylene glycol vinyl ether 98.
Proposed that important factors in controlling stereochemistry of chain growth in alkyl vinyl ether polymerization are the degree of association of growing cation and its counter anion and the bulkiness of the counter anion.
Cyclohexyl vinyl ether 98.
This oxidation is followed by mesolytic cleavage of the resulting radical cation species which leads to the generation of a reactive cation this species initiates the polymerization of the vinyl ether monomer and a dithiocarbamate radical that is likely in equilibrium with the corresponding thiuram disulfide dimer.
1 4 cyclohexanedimethanol vinyl ether mixture of cis and trans 85 technical grade.
C 10 h 18 o 2.
The photoinitiating system comprises free radical photoinitiators such as 2 2 dimethoxy 2 phenyl acetophenone dmpa benzophenone or thioxanthone together with an onium salt such as diphenyliodonium chloride and zinc bromide.
C 8 h 14 o 3.
Deprotonation with butyl lithium gives the acetyl anion equivalent.
A new photoinitiating system for living cationic polymerization of vinyl ethers is reported.
Silyl enol ethers were added to the cationic polymerization of isobutyl vinyl.
Ether method vinyl polyether cf prior art date 1998 06 08 application number pct us1999 012086 other languages french fr inventor boris a.
The toxicity of vinyl ethers has been heavily investigated because they have been used as inhalation anesthetics.
Ethyl vinyl ether also participates in inverse demand 4 2 cycloaddition reactions.
Methyl vinyl ether is an organic compound with the chemical formula ch 3 och ch 2 a colorless gas it is the simplest enol ether it is used as a synthetic building block as is the related compound ethyl vinyl ether a liquid at room temperature.
The steric repulsion of bulky substituents of terminal and penultimate monomer units could be.