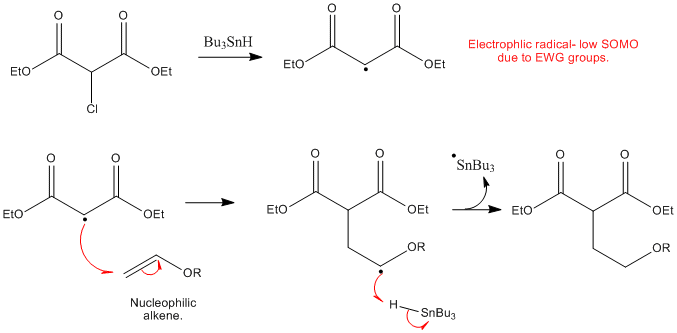
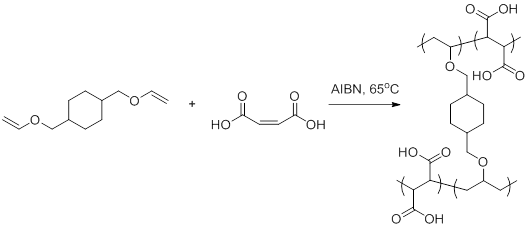
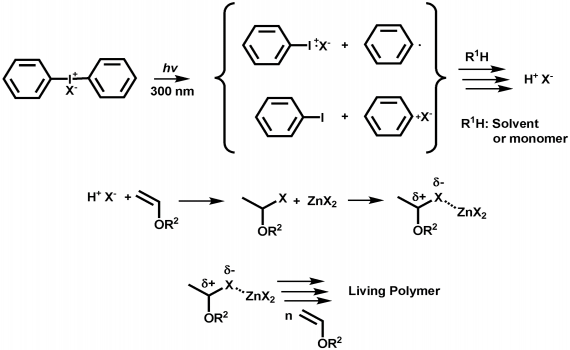
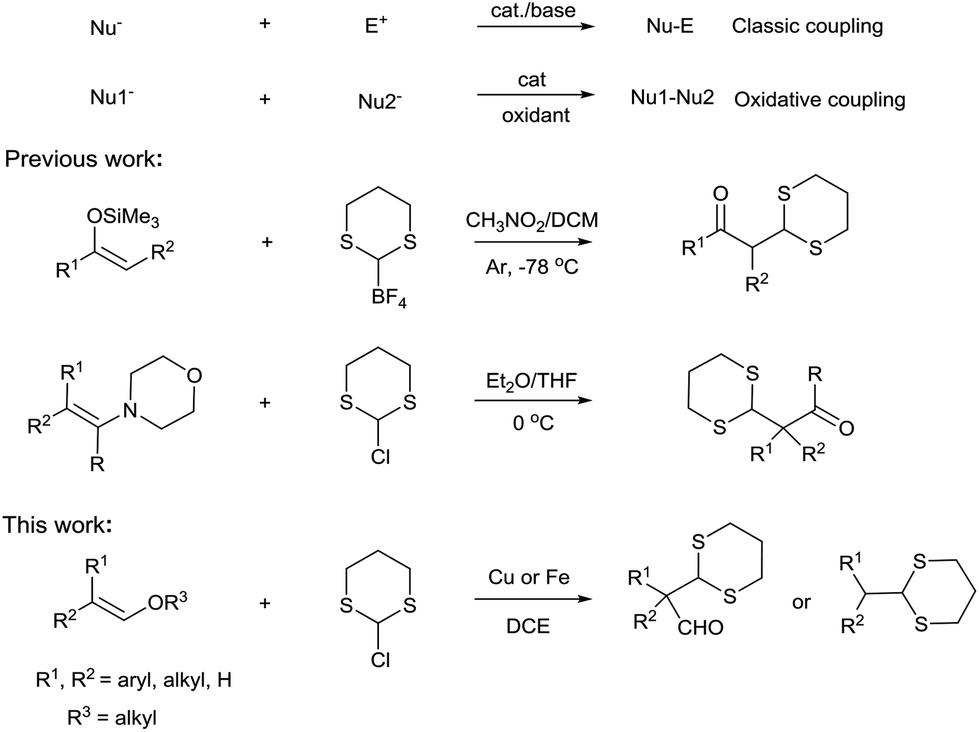
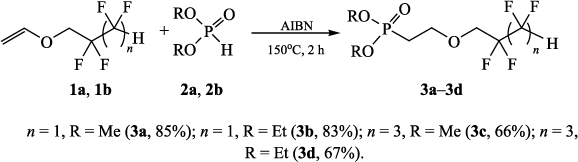
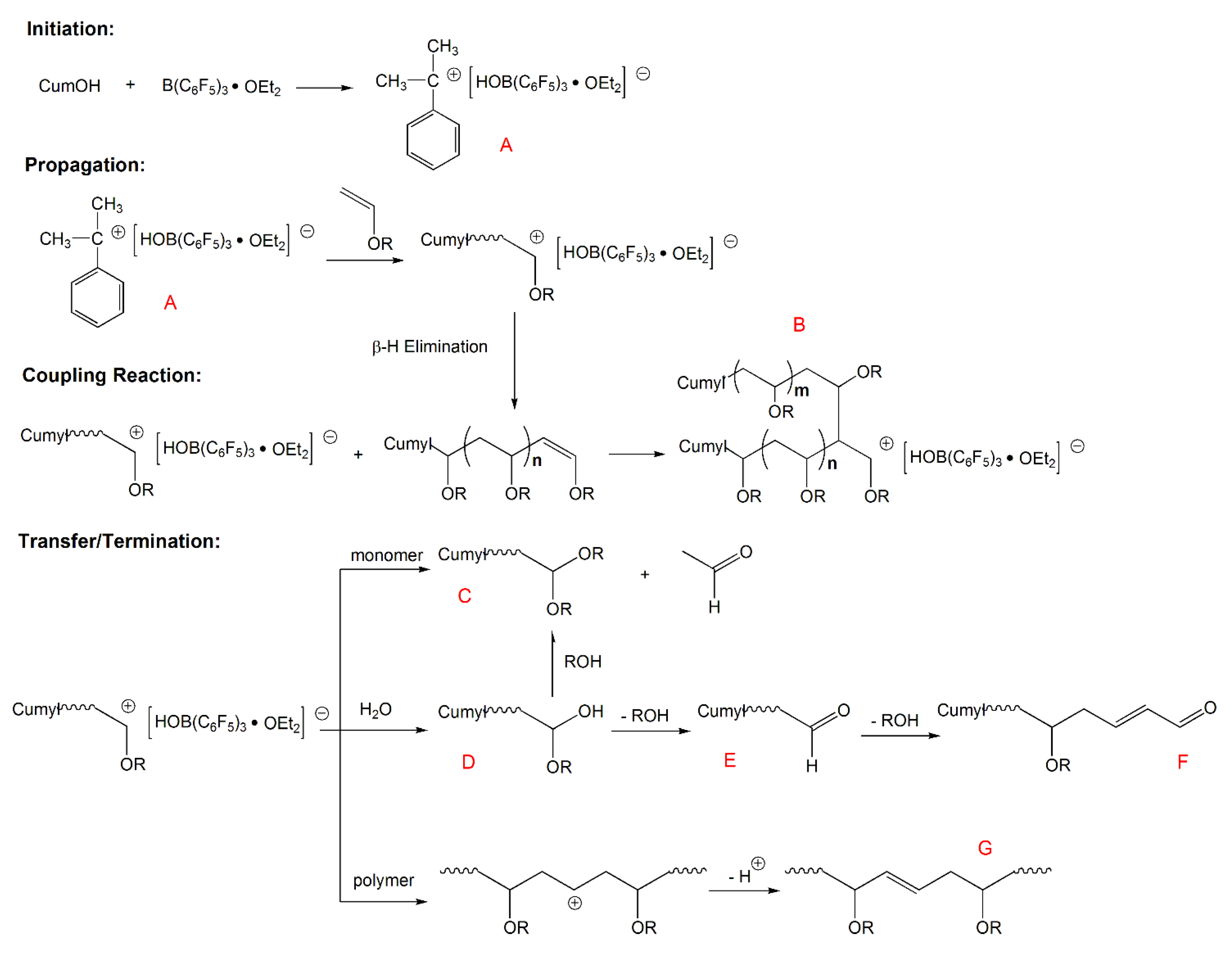
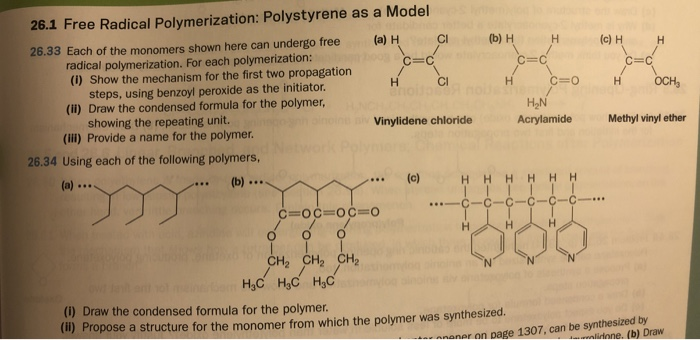
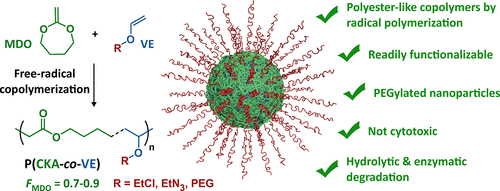
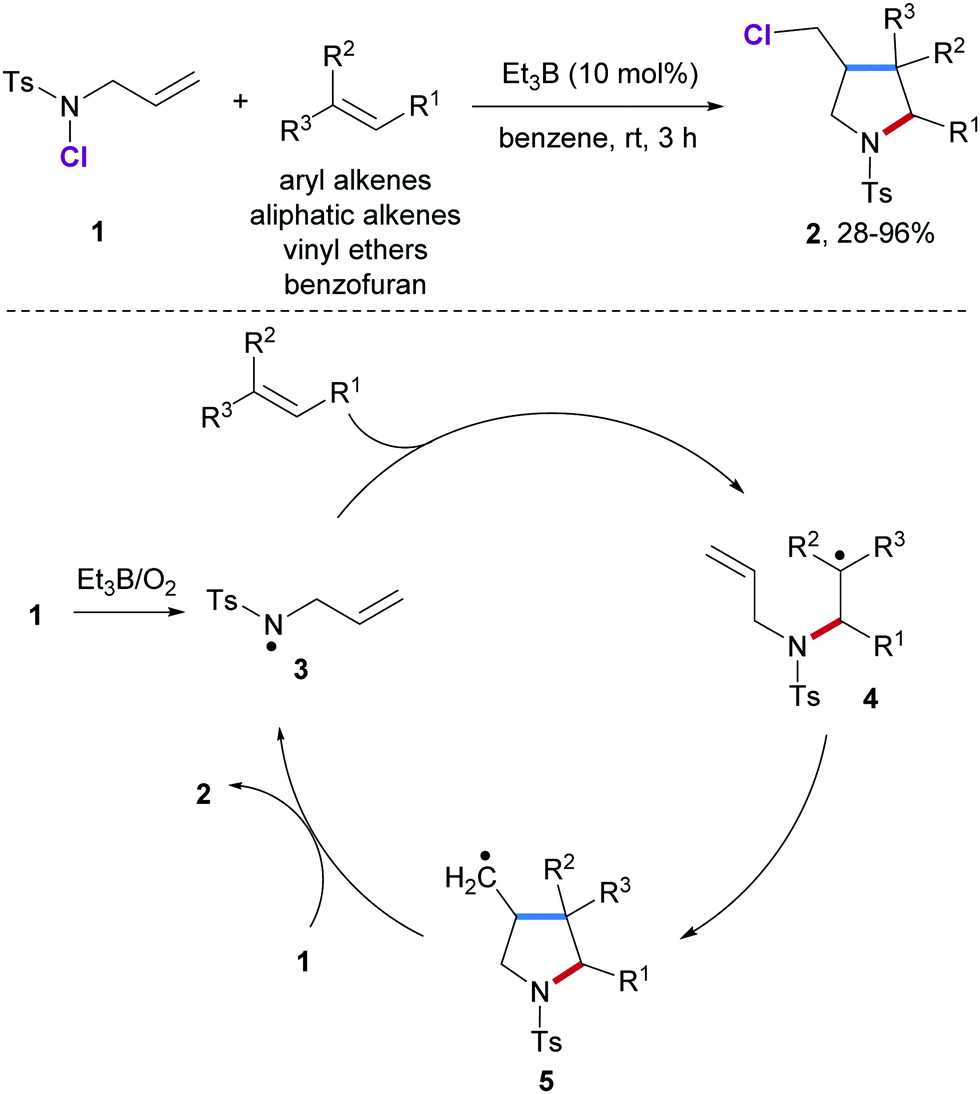
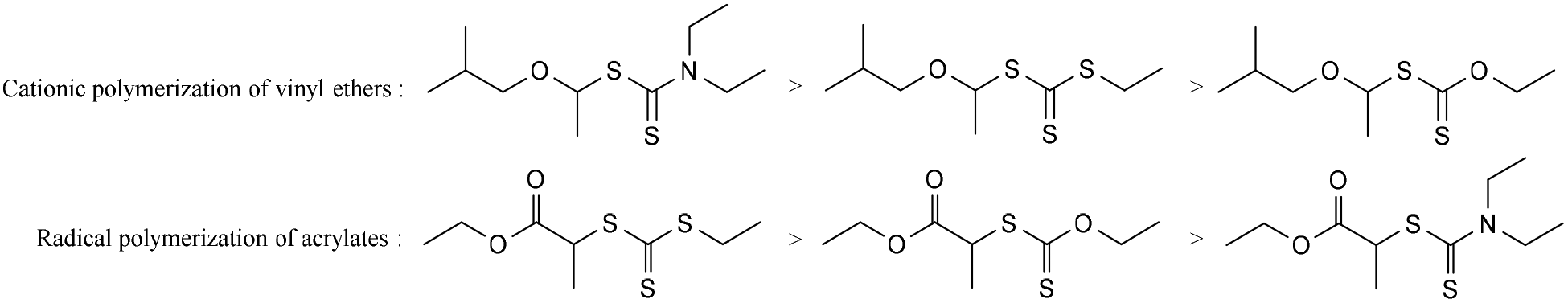
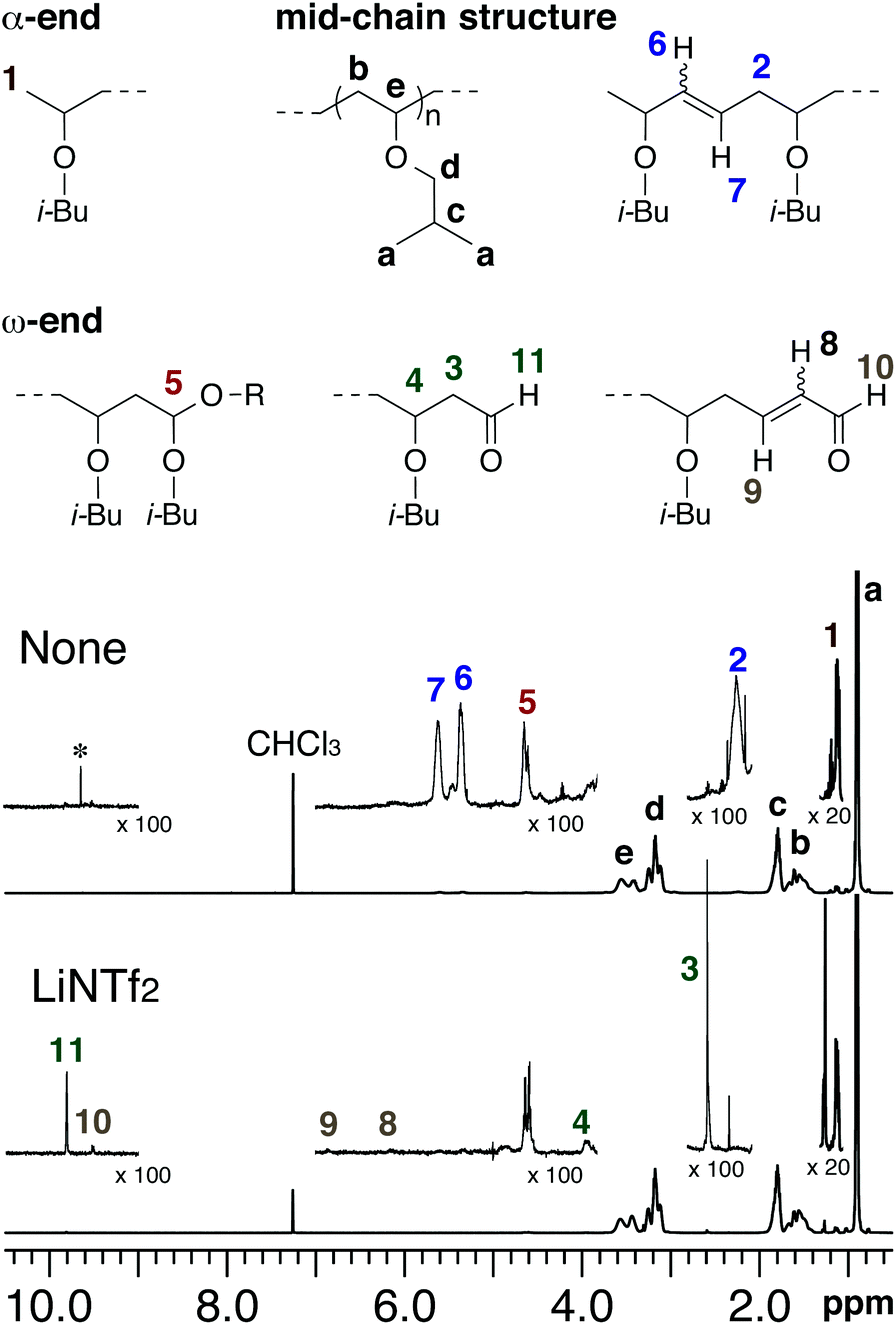
In order to overcome the challenge of direct radical polymerization of vinyl ethers commercial hydroxy functional vinyl ethers such as 2 hydroxyethyl vinyl ether heve were subjected to free radical polymerization generating.
Vinyl ethers radical.
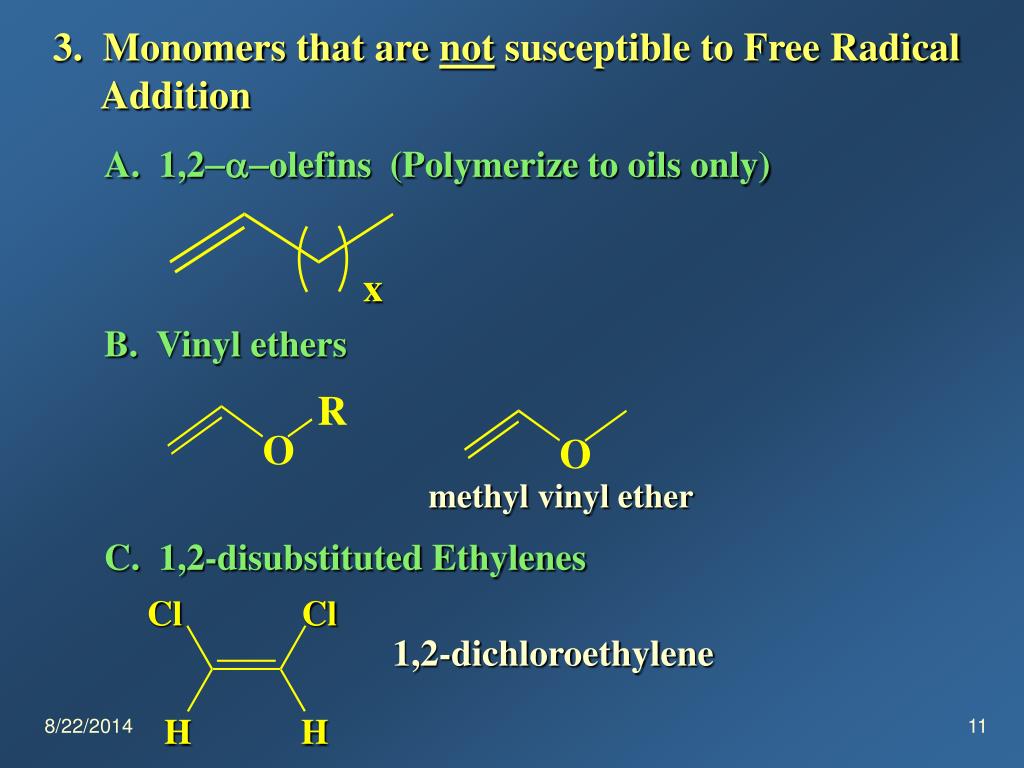
Vinyl ethers undergo homopolymerization via a cationic mechanism.
This reaction is accepted as a click chemistry reaction given the reactions high yield stereoselectivity high.
Vinyl ethers 1 which have a strongly electron donating alkoxy substituent readily form polymers on treatment with an acidic compound initiator the polymerization is thus cationic in nature.
They rely on a chiral.
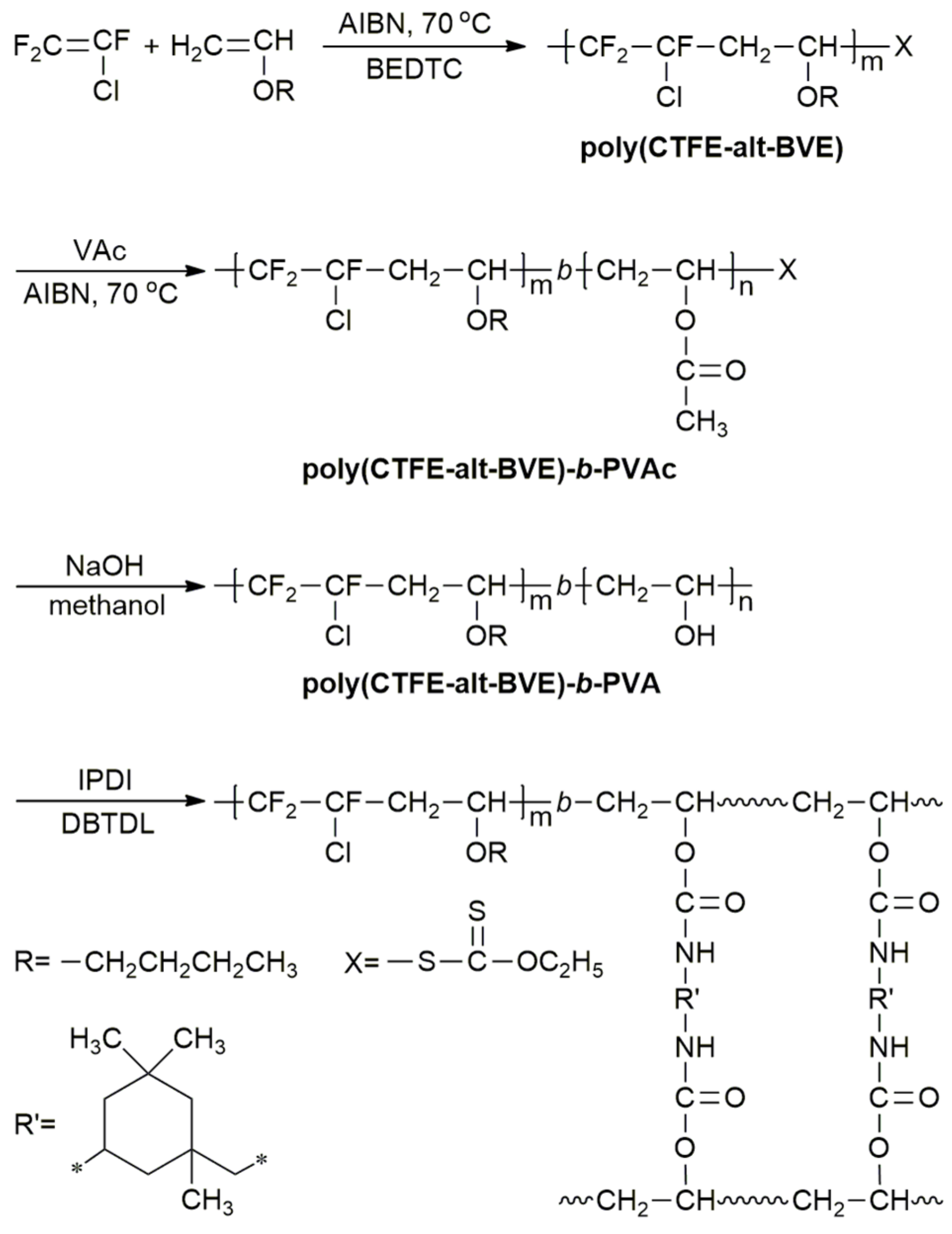
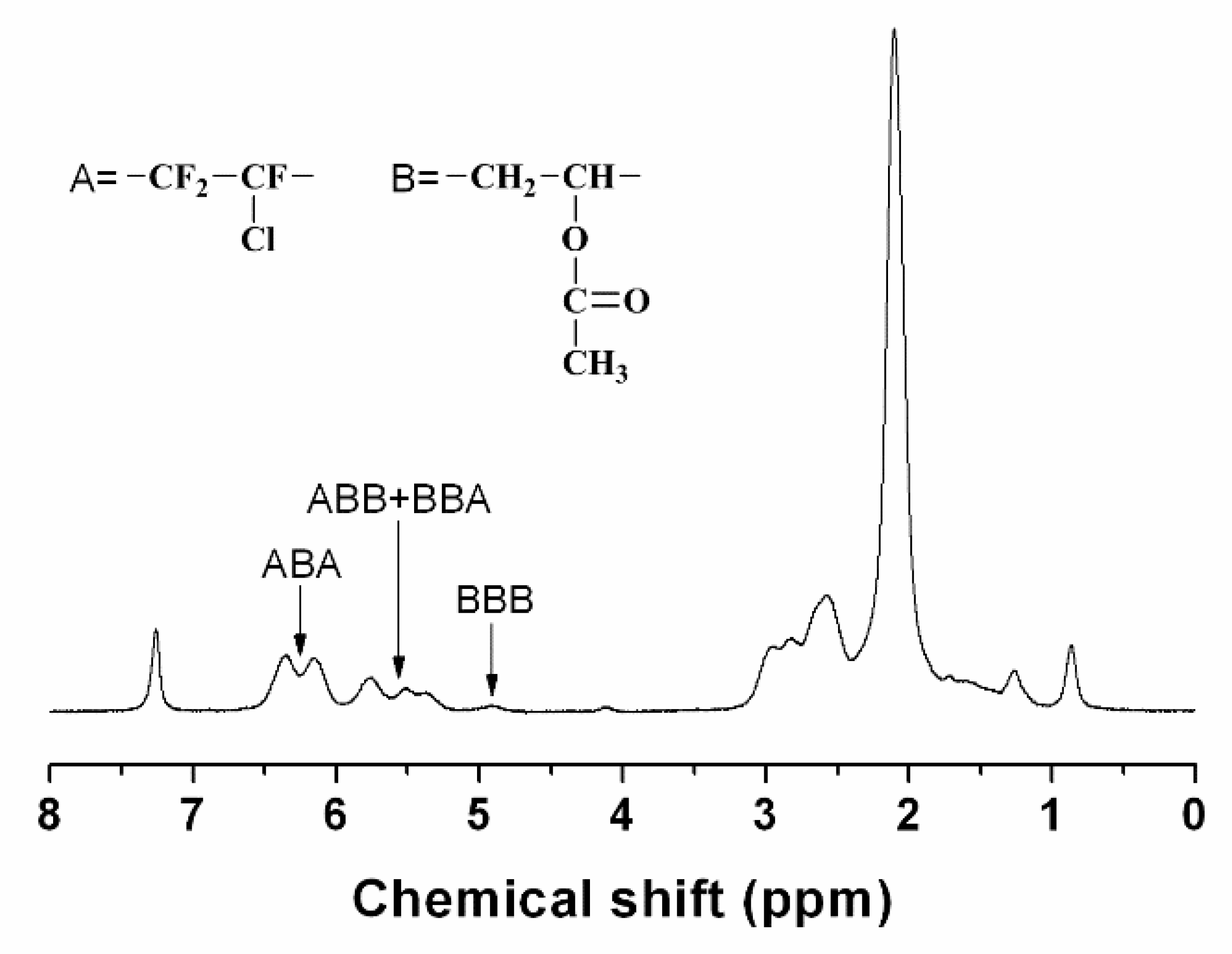
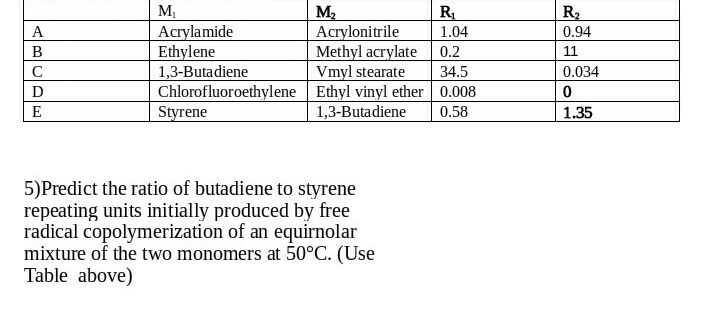
Copolymerization components include vinyl chloride vinyl acetate acrylic metacrylic acids and their ethers maleic anhydrides fluorinated olefin such as f2c cf2 and.
Well optimized catalysts produce vast quantities of isotactic polypropylene in which the side chains all face the same way.
Unprecedented controlled radical vinyl polymerization crp of vinyl ethers using reversible addition fragmentation chain transfer raft polymerization is reported.
Vinyl ether radical figure 5.
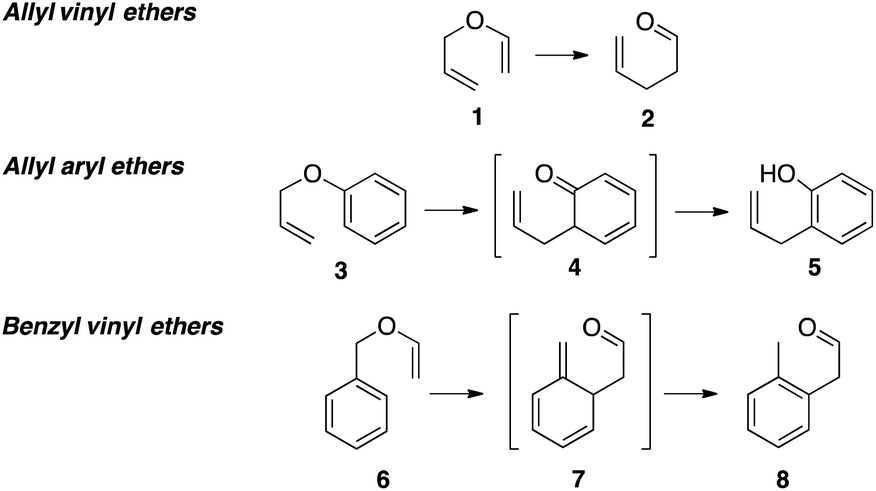
Furthermore in the presence of water they readily hydrolyse to acetaldehyde and.
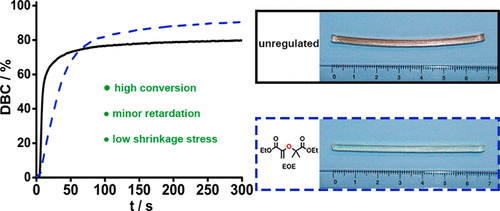
It is remarkable that only 10 addition of dve 3 increases the conversion of the acrylate from.
The product is used as low viscosity and fast curing monomers in cationic polymerization applications and as low viscosity monomers in radical polymerization applications.
It can be initiated with peroxide azo and redox initiators.
They are increasingly used in radiation curing systems because of a lower toxicity profile than the commonly used acrylic monomers.
However the polymerization under free radical conditions gives only low molecular weight oligomers and only copolymers can be synthesized.
No anionic or radical polymerizations give high polymers from vinyl ethers except for the alternating radical copolymerization with an electron deficient monomer.
Free radical copolymerization of vinyl ethers is also possible.
Teator and leibfarth report a general protocol to polymerize a variety of such vinyl ethers isotactically see the perspective by foster and o reilly.
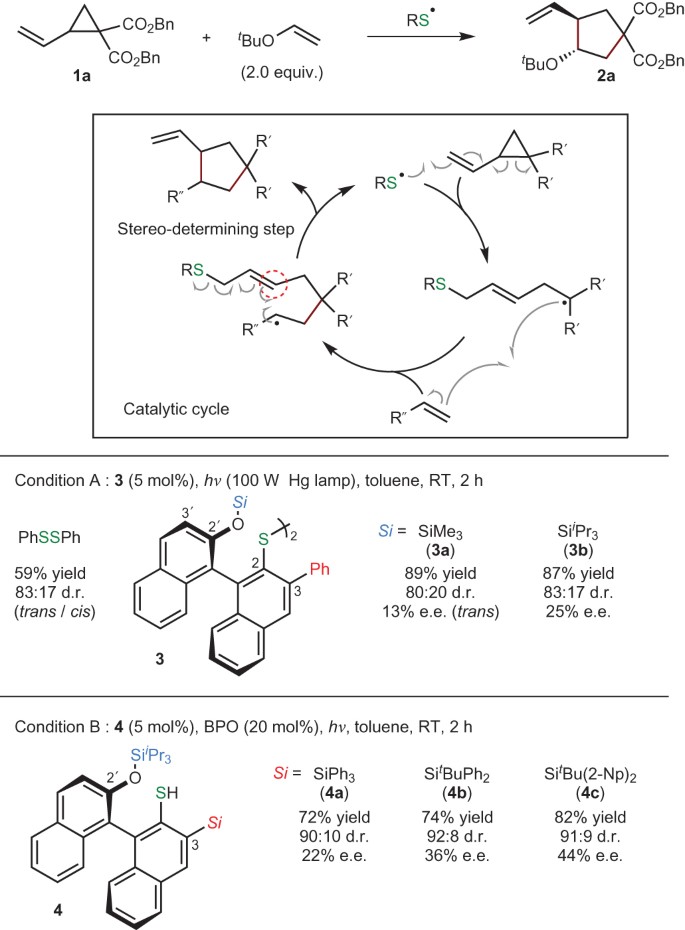
The thiol ene reaction also alkene hydrothiolation is an organic reaction between a thiol and an alkene to form a thioether this reaction was first reported in 1905 but it gained prominence in the late 1990s and early 2000s for its feasibility and wide range of applications.
Add an oxygen into the monomer though and that degree of uniformity becomes harder to enforce.