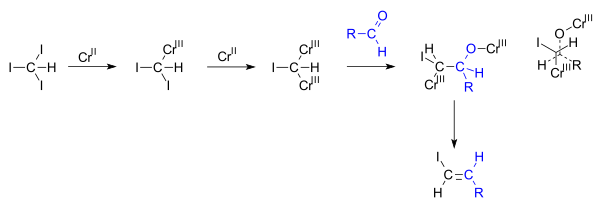
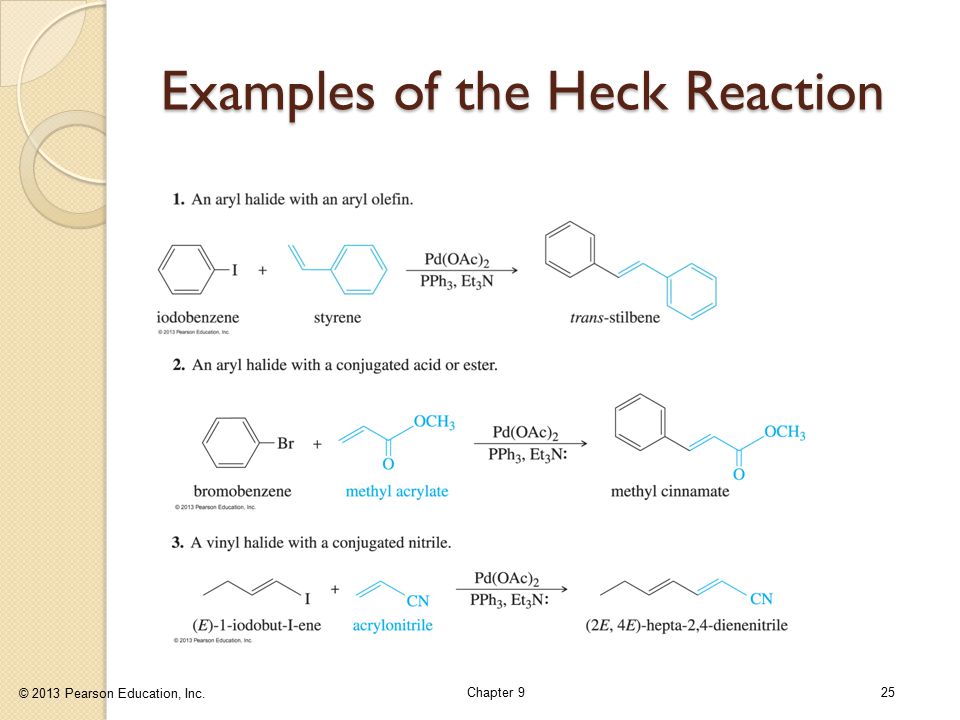
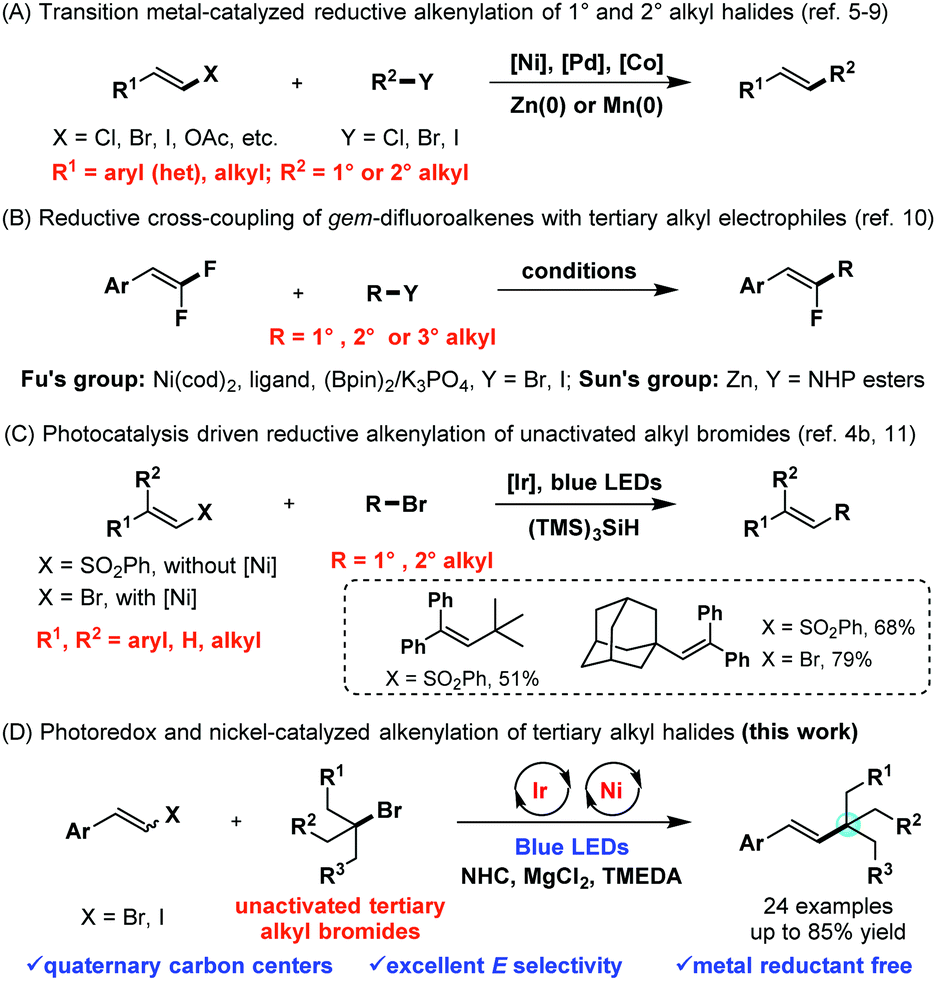
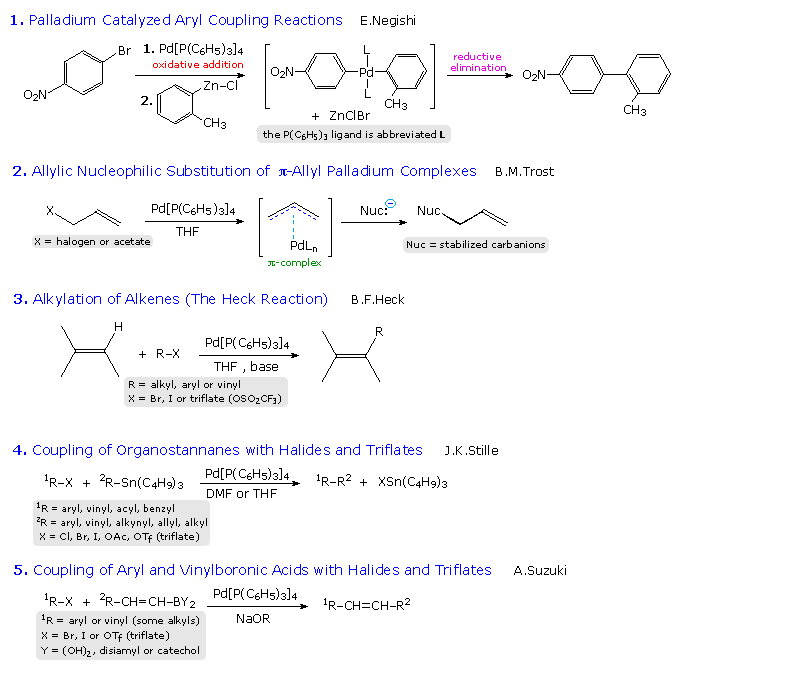
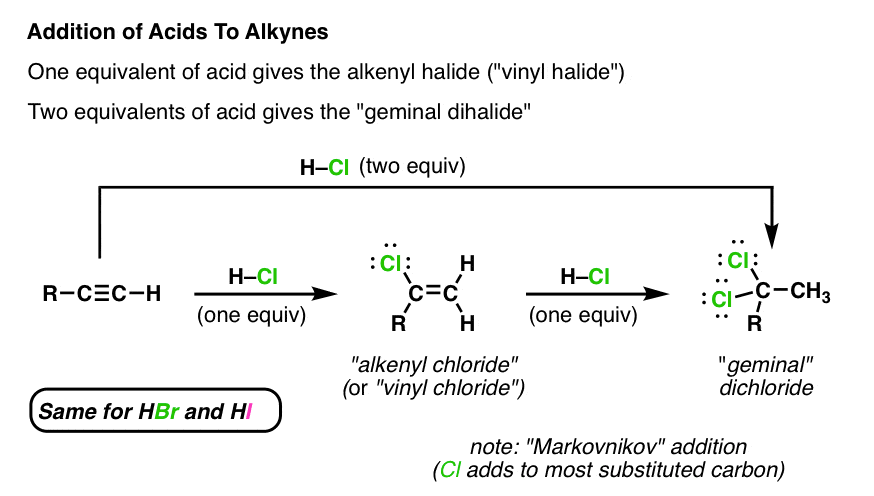However in comparison with alkyl and aryl halides the analogous utilization of vinyl halides is less developed most likely as a consequence of the highly unstable vinyl radicals generated as intermediates along with their strong tendency to abstract hydrogen atoms from a suitable source e g the solvent resulting in a synthetically less.
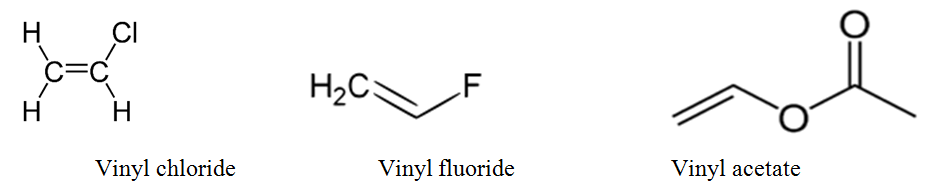
Vinyl halides examples.
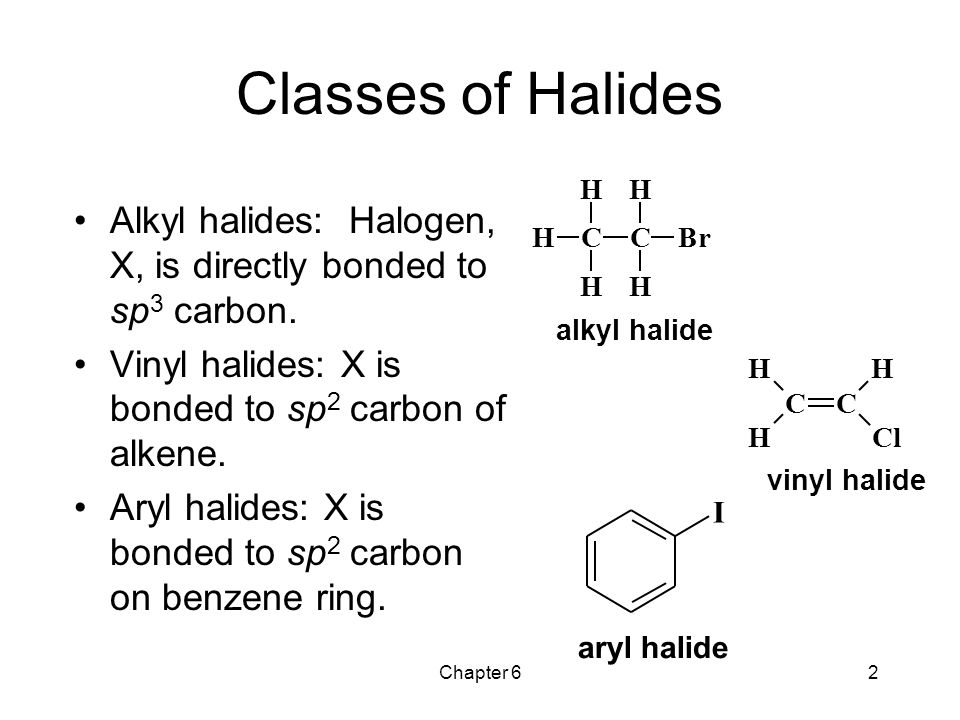
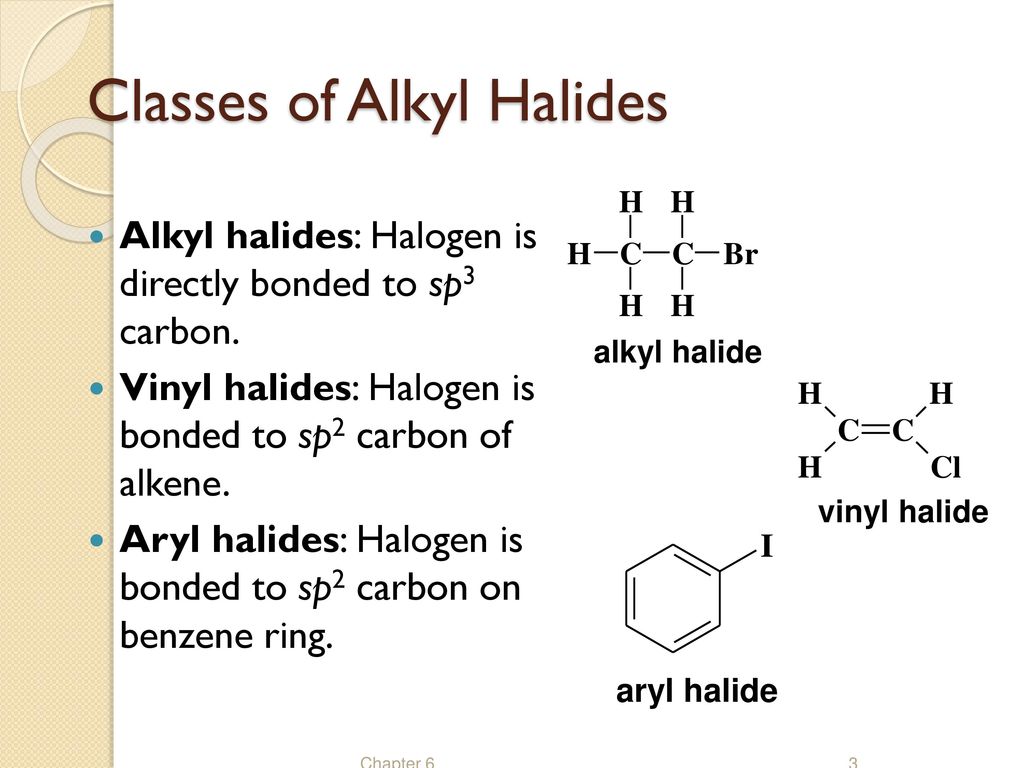
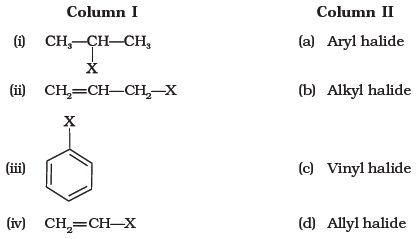
They are subdivided into alkyl vinylic aryl and acyl halides.
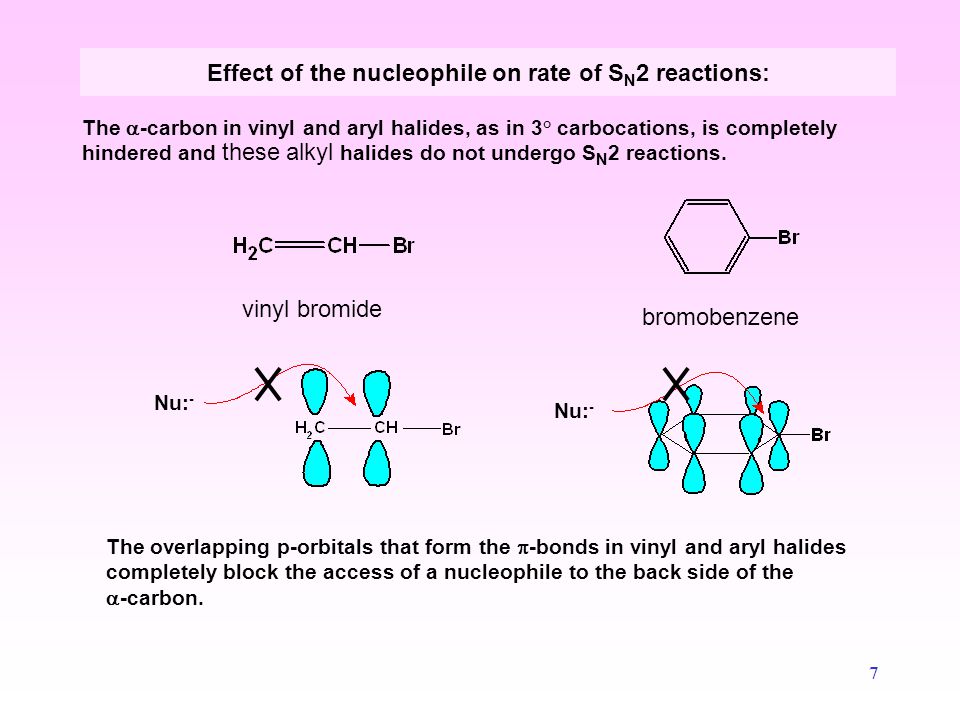
The simplest examples of an aryl halides are bromobenzene or chlorobenzene c 6h 5x.
Ethylene is a hydrocarbon with formula h c ch.
From the perspective of applications the dominant member of this class of compounds is vinyl chloride which is produced on the scale of millions of.
There are two types of vinyl halides.
Substituted vinyl halides 2.
Examples of aryl halides aryl halide structure.
An example is the addition of hydrogen chloride to vinyl chloride to yield 1 1.
From a structural.
In alkyl halides all four bonds to the carbon that bears the halogen are single bonds.
Reaction of organic halides with co and amines using stoichiometric quantities of ni co 4 or na co co 4 indicate that metal promoted amidation is a feasible route to carboxylic amides but of more use is a palladium catalyzed synthesis equation 51.
Vinylic halides may be converted to grignard reagents by reaction with magnesium and these reagents undergo the same types of reaction as those derived from alkyl halides.
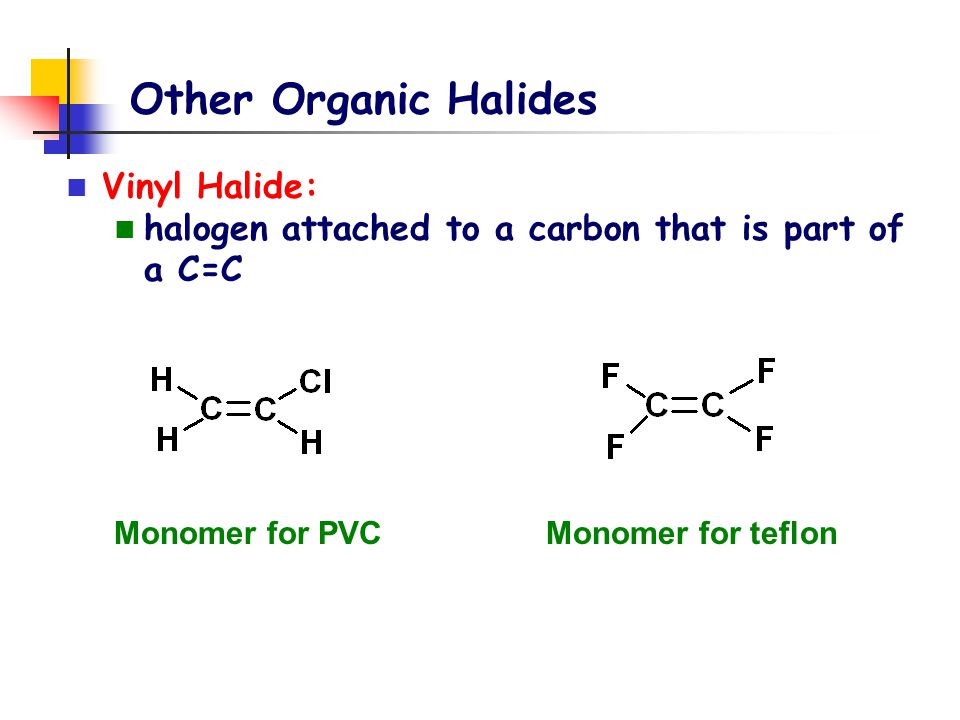
In organic chemistry a vinyl halide is a compound with the formula ch 2 chx x halide the term vinyl is often used to describe any alkenyl group.
Vinyl is basically group formed from alkene.
An aryl halide has general formula c 6h 5x in which an halide group x has substituted the aryl ring.
When we replace one hydrogen with a halogen it becomes vinyl halide.
For this reason alkenyl halides with the formula rch chx are sometimes called vinyl halides.
Vinyl halides selected introduction vinyl bromide vinyl chloride and vinyl fluoride belong to a class of structurally related chemicals referred to as simple vinyl halides or halogenated olefins these three vinyl halides are listed in the report on carcinogens as individual chemicals and not as a class.
Vinyl chloride h 2c chcl is an example.
Other articles where vinylic halide is discussed.
In vinylic halides the carbon that bears the halogen is doubly bonded to another carbon.
As we saw in the previous section an aryl halide is classified by its distinct bonding of a halogen directly to a benzene ring.