The reactions of vinyl bromides with potassium iodide proceed smoothly in the presence of a copper catalyst under mild reaction conditions to produce the corresponding vinyl iodides stereospecifically and in satisfactory to excellent yields.
Vinyl iodides synthesis.
Please select more than one item to compare.
Depending on the choice of ligand stereoselective synthesis of either cis or trans tetrasubstituted vinyl silanes is possible.
Synthesis of vinyl iodides.
This reaction represents the first example of the stereoretentive installation of multisubstitute.
Lastly vinyl iodides have been synthesised by treatment of 5 5 dialkyl n.
We also demonstrate conditions for the hiyama cross coupling of these products to prepare geometrically.
An efficient protocol for a highly stereoselective one pot synthesis of e β aryl vinyl iodides and e β aryl vinyl bromides from styrenes is based on a ruthenium catalyzed silylative coupling followed by a n halosuccinimide mediated halodesilylation reaction.
Studies on the oxidation of hydrazones with iodine and with phenylselenenyl bromide in the presence of strong organic bases.
Search results for vinyl iodide at sigma aldrich.
The fukuyama indole synthesis is a versatile tin mediated chemical reaction that results in the formation of 2 3 disubstituted indoles.
An improved procedure for the synthesis of vinyl iodides and phenyl vinyl selenides.
We report a palladium catalyzed three component carbosilylation reaction of internal symmetrical alkynes silicon electrophiles and primary alkyl zinc iodides.
Select up to 4 products.
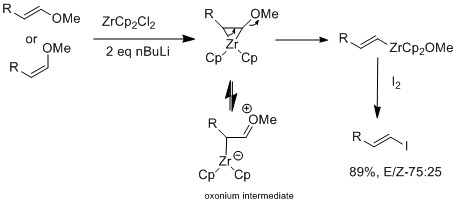
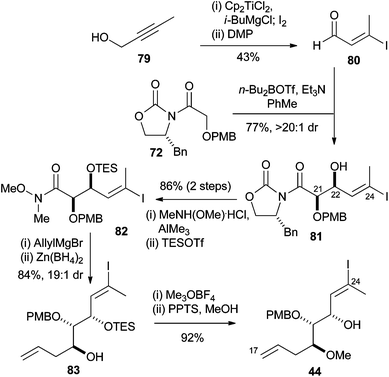
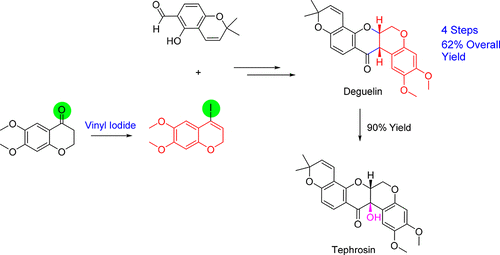
Vinyl iodides are synthesized by methods such as iodination and substitution reaction vinyl iodides with well defined geometry regiochemistry and stereochemistry are important in synthesis since many natural products and drugs that have specific structure and dimension example of regiochemistry is whether the iodide is positioned in either alpha or beta position on the olefin.
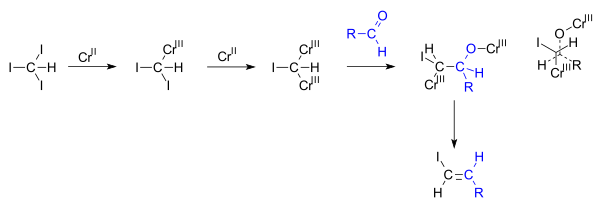
Vinyl iodides have been prepared from vinyl bromides by the action of potassium iodide and cuprous iodide in hexamethylphosphoramide hmpa 88s236.
A method is reported for palladium catalyzed n quinolyl carboxamide directed olefination of the unactivated c sp 3 h bonds of phthaloyl alanine with a broad range of vinyl iodides at room temperature.
A practical one pot reaction that can be useful for the creation of disubstituted indoles.
Most commonly tributyltin hydride is utilized as the reducing agent with azobisisobutyronitrile aibn as a radical initiator.